Fig. 2
Download original image
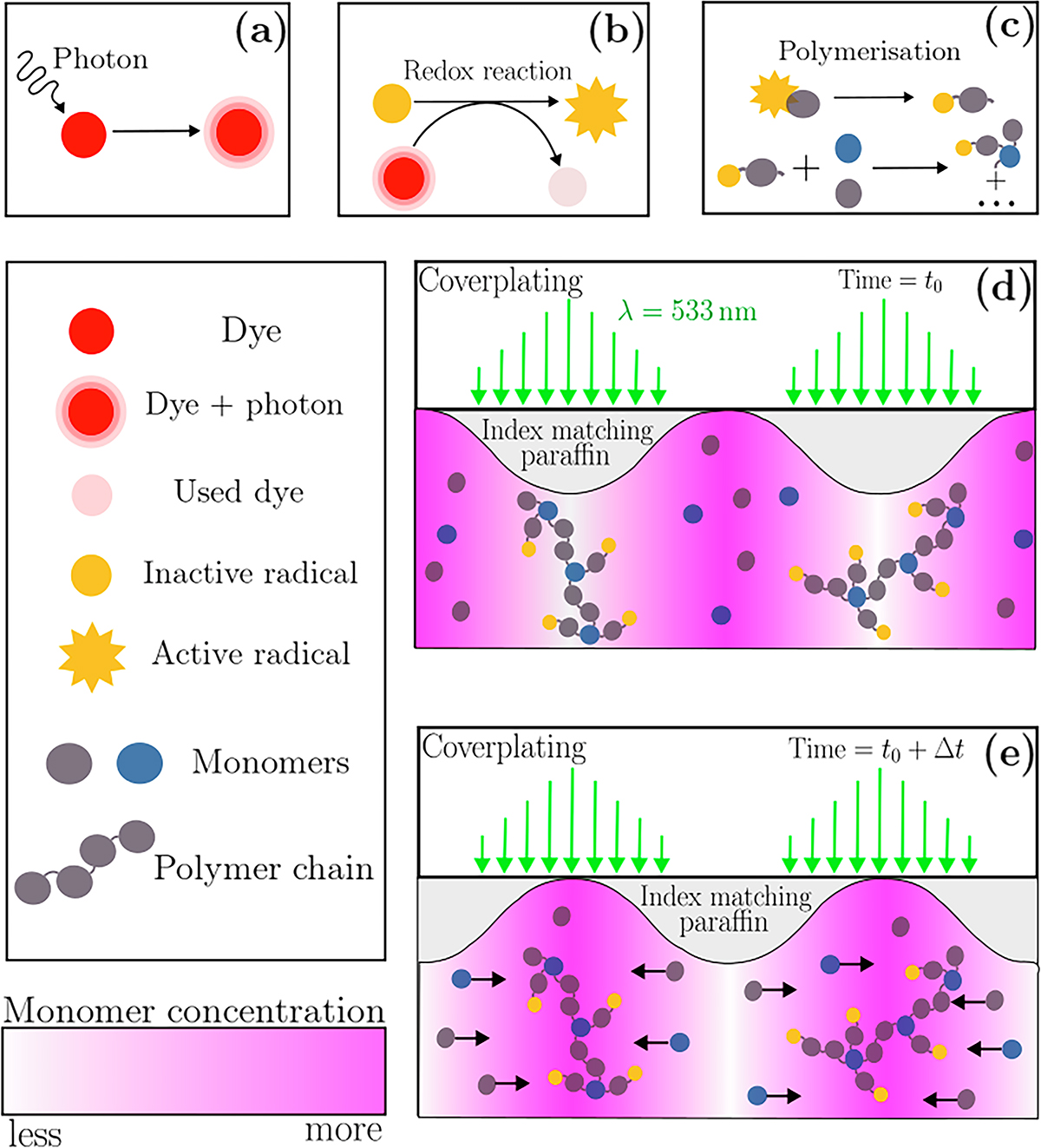
Schematic of photochemistry of photopolymerisation reaction. (a) The dye absorbs a photon from the incident light with wavelength ![]() . (b) Through a redox reaction the dye transfers its energy to the initiator, thereby activating it. (c) The photopolymerisation is initiated when the activated radical generator binds to a monomer. The generation of radicals is propagated along the chain to form a polymer chain [21]. (d) At
. (b) Through a redox reaction the dye transfers its energy to the initiator, thereby activating it. (c) The photopolymerisation is initiated when the activated radical generator binds to a monomer. The generation of radicals is propagated along the chain to form a polymer chain [21]. (d) At ![]() the photopolymerisation reaction forms a dense mesh in the illuminated areas causing shrinkage. A monomer gradient forms. (e) After a certain exposure time,
the photopolymerisation reaction forms a dense mesh in the illuminated areas causing shrinkage. A monomer gradient forms. (e) After a certain exposure time, ![]() , diffusion of monomers cause the illuminated areas to swell, as it is presented in [22]. An index matching liquid evens out the surface relief.
, diffusion of monomers cause the illuminated areas to swell, as it is presented in [22]. An index matching liquid evens out the surface relief.
Current usage metrics show cumulative count of Article Views (full-text article views including HTML views, PDF and ePub downloads, according to the available data) and Abstracts Views on Vision4Press platform.
Data correspond to usage on the plateform after 2015. The current usage metrics is available 48-96 hours after online publication and is updated daily on week days.
Initial download of the metrics may take a while.


